Rurioctocog alfa pegol
Consumer Medicine Information
What is in this leaflet
Read this leaflet carefully before you start using ADYNOVATE.
This leaflet answers some common questions about ADYNOVATE. It does not contain all
of the available information. It does not take the place of talking to your doctor
or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using
ADYNOVATE against the benefits they expect it will have for you.
If you have any concerns about having this medicine ask your doctor or pharmacist.
Keep this leaflet.
You may wish to read it again.
What is ADYNOVATE used for
ADYNOVATE belongs to the group of medicines called blood coagulation factor VIII.
It is used for the treatment and prophylaxis of bleeding in patients with haemophilia
A (congenital factor VIII deficiency).
ADYNOVATE does not contain von Willebrand factor and is therefore not suitable for
use in von Willebrand's disease.
ADYNOVATE contains the active substance rurioctocog alfa pegol, PEGylated human recombinant
coagulation factor VIII. The human coagulation factor VIII is produced by recombinant
DNA technology and has been modified chemically to prolong its duration of action.
How does ADYNOVATE work
Under normal physiological condition, factor VIII is essential for blood clotting
and maintenance of a bleeding episode.
Individuals with haemophilia A disease, which is a hereditary disorder of blood coagulation
have a low level of factor VIII in their blood circulation. As a result of factor
VIII deficiency, the individual with this disease may have a heavy bleeding into joints,
muscles or internal organs either spontaneously or as a result of accidental or surgical
trauma.
ADYNOVATE is similar to plasma-derived factor VIII. As it works in the same way, it
can be used as a replacement therapy in patients with haemophilia A.
Before you are given ADYNOVATE
ADYNOVATE should not be given to you if:
you are allergic (hypersensitive) to mouse, hamster proteins or any other ingredients
in this product;
you have tendency of allergic reaction or hypersensitivity to any human derived injection.
Some of the symptoms of allergic reaction may include skin rash, swelling of the face,
lips or tongue, which may cause difficulty swallowing or shortness of breath, tightness
of the chest;
the expiry date printed on the pack has passed;
the packaging is torn or show sign of tampering;
the pack has been stored at room temperature for more than 3 months.
You must tell your doctor if you:
have any other illness;
suffer from, or at risk of, any heart problems or any disease and/or conditions involving
the blood vessels;
are on a low sodium diet.
Tell your doctor if you are pregnant or planning to become pregnant.
There is no information on the use of ADYNOVATE during pregnancy. Your doctor will
discuss the risks and benefits of using ADYNOVATE if you are pregnant.
Tell your doctor if you are breast feeding or planning to breast feed.
It is not known whether ADYNOVATE passes into breast milk. Your doctor will discuss
the risks and benefits of using ADYNOVATE if you are breast feeding.
If you have not told your doctor any of the above, tell your doctor before you start
using ADYNOVATE.
Taking other medicines
Tell your doctor or pharmacist or Haemophilia Treatment Centre if you are using any
other medicines including any that you obtained without a prescription from your pharmacy,
supermarket or health food shop.
No drug interactions have been reported with ADYNOVATE, but your doctor or pharmacist
or Haemophilia Treatment Centre will have more information on medicines to be careful
with or avoid while using this medicine.
How ADYNOVATE is given
How much is given
Your doctor will decide how much ADYNOVATE will be given to you depending on your
need and condition. Each individual will receive a different dose, which may vary
between doctor visits.
The dose you receive will be based on:
your body weight;
the amount of antihaemophilic factor (AHF) your body is able to make;
how much and how often and which sites (knees, muscles etc.) of your body are bleeding;
whether your body may build up antibodies to this medicine. After a while your body
may build up these antibodies, leading to a less effective treatment than the usual.
How it is given
ADYNOVATE is usually administered in a hospital. Some individuals may be trained to
use ADYNOVATE at home.
ADYNOVATE is given by a slow injection directly into your vein.
Do not attempt to inject ADYNOVATE by yourself unless you have received proper training
on how to use the product by your doctor or other competent healthcare professional,
e.g. a haemophilia nurse.
If you are unsure about how to use this medicine, contact your doctor or competent
healthcare professional to seek advice.
Method of administration (use aseptic technique)
Aseptic (meaning clean and germ free) technique is required for the handling, preparing
and administration of ADYNOVATE.
ADYNOVATE is provided as a powder and water for injections
Follow carefully the instructions for use given at the end of this leaflet for preparing
the solution for injection, and for injecting ADYNOVATE.
Ask your doctor or Haemophilia Treatment Centre for help if you do not understand
the instructions given in this leaflet.
Use only the reconstitution device provided with each pack to prepare the solution
for injection.
Do not mix ADYNOVATE with any other medicines or solvent other than the water for
injections supplied with the pack.
After preparing the solution, use the solution straight away or within 3 hours.
Do not refrigerate the solution after it is prepared.
Before injection, check the solution to make sure it is clear, colourless and free
of any particles. Do not use if the solution contains particles or looks cloudy.
Use a new syringe and needle for each injection.
How often is it given
Your doctor will tell you how often and what intervals ADYNOVATE is to be administered.
If you miss / forget your injection
Do not inject a double dose to make up for the forgotten dose.
If you inject a double dose, it will increase the chance of you getting an unwanted
side effect.
Contact your doctor or Haemophilia Treatment Centre for advice and give the next injection
as advised by your doctor.
If you use too much (overdose)
No symptoms of overdose with ADYNOVATE have been reported.
Immediately telephone your doctor or the Poisons Information Centre (telephone 131
126), or go to accident and emergency at your nearest hospital, if you think that
you or anyone else may have been given too much ADYNOVATE.
Do this even if there are no signs of discomfort or poisoning.
While you are using ADYNOVATE
Things you must do
Continue using your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it.
Keep all your doctor’s appointments so that your progress can be checked.
Discuss with your doctor the progress you have experienced after the treatment, especially
during the first few days.
Your healthcare professional will take records of the progress and unexpected reactions.
You will have your blood tested before you start your treatment and regularly from
time to time during your treatment to see how your treatment is working.
Monitor your bleeding and tell your doctor immediately if your bleeding is not controlled
after using ADYNOVATE.
Take caution when you receive the injection, and tell your doctor or nurse immediately
if you feel unwell during the injection. If you are giving the injection by yourself,
stop the injection immediately if you feel unwell.
Allergic reaction may occur during an injection.
If you are planning for any surgery or dental procedures, tell your doctor and dentist
that you are using ADYNOVATE.
If you require a central venous access device, your doctor will need to consider the
risk of catheter-related complications including infection at the catheter site, presence
of bacteria in the blood and blood clot at the catheter site.
Things you must not do
Do not give ADYNOVATE to anyone else even if they appear to have the same condition
as you.
Do not stop using ADYNOVATE or change the dose without checking with your doctor,
unless you have an allergic reaction.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you
are using ADYNOVATE.
All medicines can have side effects. Sometimes they are serous, most of the time they
are not.
You may need medical attention if you get some of these side effects.
Do not be alarmed by this list of possible side effects, you may not experience any
of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor, nurse or pharmacist if you notice any of the following and they
worry you:
headache
The above list includes the very common side effects of ADYNOVATE.
dizziness
nausea
diarrhoea
rash
hives
The above list includes the common side effects of ADYNOVATE.
flushing
redness of the eye
allergic reactions
development of factor VIII inhibitors
increase in some type of white blood cells
skin reactions triggered by the medicine
skin reactions at injection site
The above list includes the uncommon side effects of ADYNOVATE.
If any of the following happen suddenly, tell your doctor immediately or go to Emergency
at the nearest hospital:
rash, hives, wheals, generalised itching;
swelling of your face, lips and tongue or any parts of the body;
shortness of breath, difficulty in breathing, wheezing, tightness in the chest,
general feeling of being unwell,
dizziness and loss of consciousness.
The above list includes serious side effects which are early symptoms of an allergic
response to the medicine. Allergic reaction (hypersensitivity) is an uncommon side
effect of ADYNOVATE, which in some cases could become serious (progressing to anaphylaxis
including shock) and require urgent medical attention or hospitalisation. If any of
the above occurs during the ADYNOVATE injection, stop the injection immediately.
During the course of your treatment, your body could make antibodies (also called
"inhibitors") against ADYNOVATE, and stop ADYNOVATE from working properly.
Tell your doctor or Haemophilia Treatment Centre if you notice anything that is making
you feel unwell.
After using ADYNOVATE
Storage
All medicines should be kept where children cannot reach them.
Store ADYNOVATE in the refrigerator at 2°C to 8°C.
Do not freeze ADYNOVATE.
Keep ADYNOVATE in the pack until it is time to use it.
This will protect the medicine from light.
Unopened vials stored in the pack can be kept in the refrigerator at 2°C to 8°C until
its expiry date which is printed on the packaging.
If necessary, ADYNOVATE can be kept in the sealed carton out of the refrigerator for
a single period of up to 3 months when stored in a cool dry place where the temperature
stays below 30°C.
Do not return the product to the refrigerator after it has been storage at room temperature.
Use ADYNOVATE within 3 months if stored at room temperature.
The medicine expires 3 months after the pack is stored at room temperature, or after
the expiration date printed on the carton has passed, whichever is earlier.
On the day the product is removed from the refrigerator, write the discard date on
the carton to remind yourself to discard the product after 3 months.
Once the solution is prepared for injection, use the solution straight away or within
3 hours after it is prepared.
Do not refrigerate the solution in after it is prepared.
Disposal
Discard any solution left in the vial at the end of your injection.
ADYNOVATE is for single use in single patient only. It does not contain antimicrobial
preservative.
Dispose the used vials and all materials in an appropriate container.
If your doctor tells you to stop using ADYNOVATE or the expiry date has passed, ask
your Haemophilia Treatment Centre what to do with any medicine that is left over.
Ask your doctor or Haemophilia Treatment Centre if you have any questions about how
to dispose ADYNOVATE.
Product descriptions
What ADYNOVATE looks like
ADYNOVATE is a white to off-white friable powder in a single dose glass vial.
Each pack contains:
1 vial of ADYNOVATE powder for injection;
1 vial of water for injections (which is a diluent to dissolve the powder for injection);
1 BAXJECT II Hi-Flow or BAXJECT III (preassembled) reconstitution device.
After reconstitution, the solution is clear, colourless and free from foreign particles.
ADYNOVATE is available in the following strengths, which may be supplied with either
a 5 mL water for injections vial or a 2 mL water for injections vial:
250 IU (5 mL or 2 mL);
500 IU (5 mL or 2 mL);
750 IU (5 mL or 2 mL);
1000 IU (5 mL or 2 mL);
1500 IU (5 mL or 2 mL);
2000 IU (5 mL); and
3000 IU (5 mL).
Ingredients
Active ingredient:
rurioctocog alfa pegol (Recombinant Coagulation Factor VIII (rch), PEGylated)
Inactive ingredients:
calcium chloride dihydrate;
glutathione;
histidine;
mannitol;
polysorbate 80;
sodium chloride;
trehalose dihydrate;
trometamol;
water for injections (diluent).
Sponsor
ADYNOVATE is supplied in Australia by:
Takeda Pharmaceuticals Australia Pty Ltd
Level 39, 225 George Street
Sydney NSW 2000
Australia
Telephone: 1800 012 612
ADYNOVATE is supplied in New Zealand by:
Takeda New Zealand Limited
Level 10, 21 Queen Street
Auckland 1010
Telephone: 0508 169 077
Australian registration numbers
AUST R 273517
(ADYNOVATE 250 IU)
AUST R 278727
(ADYNOVATE 500 IU)
AUST R 300850
(ADYNOVATE 750 IU)
AUST R 278728
(ADYNOVATE 1000 IU)
AUST R 300851
(ADYNOVATE 1500 IU)
AUST R 278729
(ADYNOVATE 2000 IU)
AUST R 300852
(ADYNOVATE 3000 IU)
Date of preparation
This leaflet was prepared in March 2022.
Instructions for use
For intravenous use only
IMPORTANT:
Contact your doctor or local Haemophilia Treatment Centre if you have any questions
or if you experience any problems with this procedure.
These instructions are intended only as a visual aid for those patients who have been
instructed by their doctor or Haemophilia Treatment Centre on the proper way to self-inject
the product.
Do not attempt to self-inject unless you have been taught how by your doctor or Haemophilia
Treatment centre.
Use only the water for injections and the reconstitution device provided in the pack
to prepare the solution for injection.
ADYNOVATE is supplied either with a BAXJECT II Hi-Flow or a BAXJECT III (preassembled)
reconstitution device.
Please refer to the instructions corresponding to the device supplied with your ADYNOVATE,
as illustrated below.
If more than one vial of ADYNOVATE is needed for the dose, reconstitute each vial
of ADYNOVATE using a separate BAXJECT II Hi-Flow or a separate preassembled BAXJECT
III system supplied in each pack.
Use aseptic technique
In a quiet place, prepare a clean surface and gather all the materials you will need
for the injection.
Remove ADYNOVATE from the refrigerator and check the expiry date on the package.
Wash your hands and put on clean exam gloves.
If you are self-injecting at home the use of gloves is optional.
Using the BAXJECT II Hi-Flow Device
1. Use aseptic technique (clean and germ free) and a flat work surface during the reconstitution
procedure.
2. Allow the ADYNOVATE powder and diluent vials to reach room temperature before use.
3. Remove plastic caps from the ADYNOVATE powder and diluent vials.
4. Cleanse rubber stoppers with an alcohol wipe and allow to dry before use.
5. Open the BAXJECT II Hi-Flow device package by peeling away the lid, without touching
the inside (Figure A).
Do not remove the device from the package.
Figure A
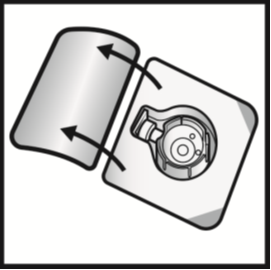
6. Turn the package over.
Press straight down to fully insert the clear plastic spike through the diluent vial
stopper (Figure B).
Figure B
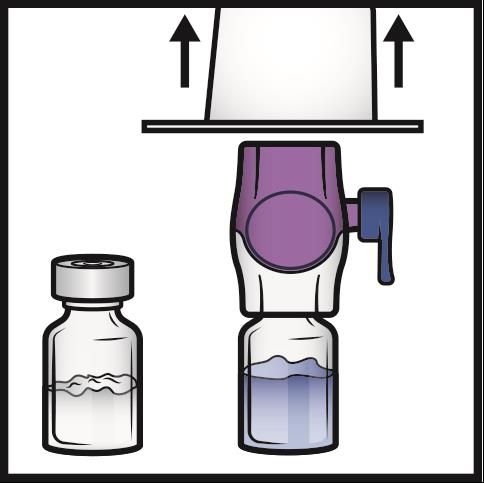
7. Grip the BAXJECT II Hi-Flow package at its edge and pull the package off the device
(Figure C).
Do not remove the blue cap from the BAXJECT II Hi-Flow device.
Do not touch the exposed purple plastic spike.
Figure C
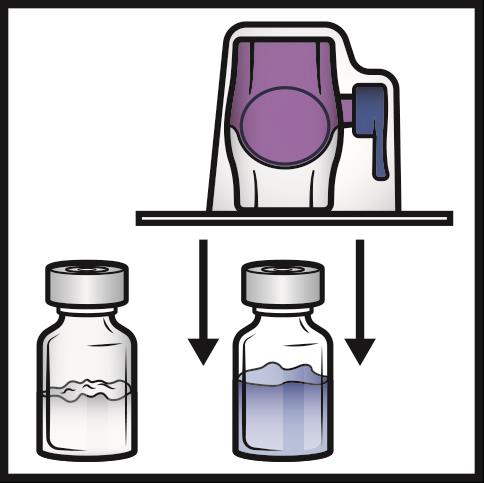
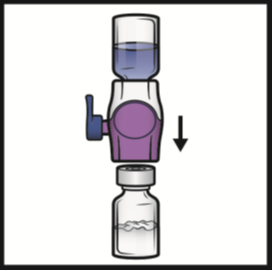
8. Turn the system over so that the diluent vial is on top.
Quickly insert the purple plastic spike fully into the ADYNOVATE powder vial stopper
by pushing straight down (Figure D).
The vacuum will draw the diluent into the ADYNOVATE powder vial.
Figure D
9. Swirl gently until the ADYNOVATE powder is completely dissolved.
The powder should dissolve rapidly (usually in less than 1 minute).
Do not refrigerate the solution after reconstitution.
Administration
Use the reconstituted solution as soon as possible, within 3 hours after reconstitution.
Before administering the dose, visually inspect the reconstituted solution for particulate
matter and discoloration.
The solution should appear clear and colourless.
Do not use the solution if there is any particulate matter or discoloration.
1. Remove the blue cap from the BAXJECT II Hi-Flow device (Figure E).
Connect the syringe to the BAXJECT II Hi-Flow.
Use of a Luer-lock syringe is recommended.
Figure E
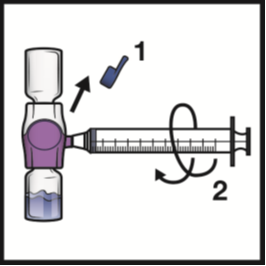
2. Turn the system upside down (ADYNOVATE powder vial now on top).
Draw the reconstituted solution into the syringe by pulling the plunger back slowly
(Figure F).
Do not draw air into the syringe.
Figure F
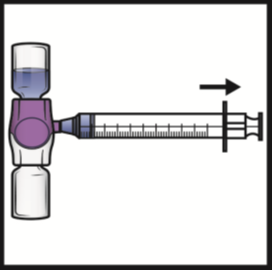
3. Disconnect the syringe; attached a needle suitable for intravenous injection.
If more than one vial of ADYNOVATE is to be used, the contents of multiple vials may
be drawn into the same syringe.
4. Administer the dose by injecting the solution directly into the vein over a period
of up to 5 minutes (maximum infusion rate of 10 mL per min).
Using the BAXJECT III system
The ADYNOVATE powder vial and the diluent vial are supplied preassembled with the
BAXJECT III reconstitution device within a sealed blister package.
Do not use if the lid is not completely sealed on the blister packaging.
1. Take the sealed blister out from the pack and allow the ADYNOVATE powder and diluent
vials to reach room temperature before use.
2. Open the blister package by peeling away the lid. Remove the preassembled BAXJECT
III system from the blister.
3. Place the pre-assembled BAXJECT III system on a flat surface with the diluent vial
on top (Figure 1). The diluent vial has a blue stripe.
Do not remove the blue cap until instructed in a later step.
Figure 1
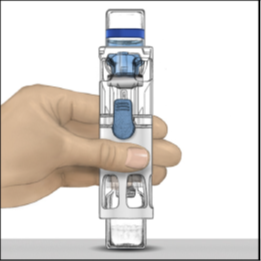
4. Hold the lower end of the pre-assembled BAXJECT III system with one hand, and use
the other hand to press down firmly on the diluent vial until the system is fully
collapsed and the diluent flows down into the ADYNOVATE powder vial (Figure 2).
Do not tilt the system until all the diluent is completely transferred to the ADYNOVATE
powder vial.
Figure 2
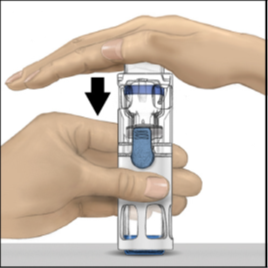
5. Check to make sure the diluent transfer is complete. Swirl gently until all the ADYNOVATE
powder is dissolved (Figure 3).
The powder should dissolve rapidly (usually in less than 1 minute).
Figure 3
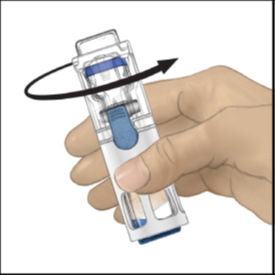
Make sure all the ADYNOVATE powder is completely dissolved, otherwise the undissolved
powder will not pass through the device filter.
Do not refrigerate the solution after reconstitution.
Administration
Use the reconstituted solution as soon as possible, within 3 hours after reconstitution.
Before administering the dose, visually inspect the reconstituted solution for particulate
matter and discoloration before administration.
The solution should appear clear and colourless.
Do not use the solution if there is any particulate matter or discoloration.
1. Remove the blue cap from the BAXJECT III device. Connect the syringe to the BAXJECT
III device. Use of a Luer-lock syringe is recommended.
2. Turn the system upside down (ADYNOVATE powder vial now on top). Draw the reconstituted
solution into the syringe by pulling the plunger back slowly. Do not draw air into
the syringe.
3. Disconnect the syringe; attach a needle suitable for intravenous injection.
If more than one vial of ADYNOVATE is to be used, the contents of multiple vials may
be drawn into the same syringe.
4. Administer the dose by injecting the solution directly into the vein over a period
of up to 5 minutes (maximum infusion rate 10 mL per min).
ADYNOVATE® and BAXJECT® are registered trademarks of Baxalta Incorporated.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.