Biosimilars are a form of biological drugs “highly similar” to a biologic approved earlier, which is also called a reference innovator product.
It is crucial for a biosimilar to undergo stringent similarity testing at every stage of its development to show that potential variations from the reference product are not clinically meaningful concerning the quality, safety and efficacy (EMA), or safety, purity and potency (FDA).
The binding between a biosimilar and its target antigen (and Fc receptors) is regarded as a key indicator of its efficacy. ACROBiosystems has designed high-quality target antigen proteins and Fc receptor proteins to enable biosimilar research.
Product list
Target antigen proteins
- TNF-alpha
- Her2
- VEGF165
- EDF R
- CD 20
- VEGF 121
- RANKL
- PD-1
- PD-L1
- CTLA4
Fc receptor proteins
- FcRn
- Fc gamma RIIIA/CD16a
- Fc gamma RIIIB/CD16b
- Fc gamma RIIA/CD32a
- Fc gamma RIIB/CD32b
- Fc gamma RI/CD64
Why choose ACROBiosystems?
Validated with reference innovator drugs
A majority of the target antigens and Fc receptors from ACROBiosystems have been validated with the reference innovator drugs in functional ELISA, SPR or BLI. The reference innovator drugs are Herceptin®, Avastin®, MabThera®, Erbitux® and Humira®, which are bought from Roche, Merck KGaA and AbbVie.
Her2 antigen-binding affinity was determined by ELISA using Trastuzumab (Herceptin®)
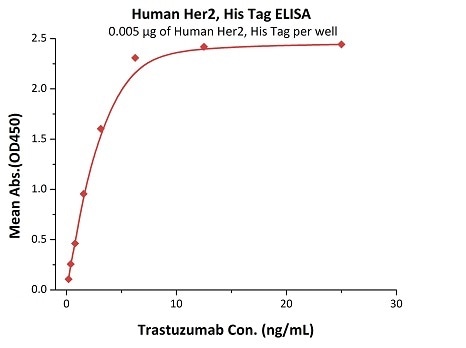
Immobilized Human Her2, His Tag (Cat. No. HE2-H5225) at 0.05 μg/mL (100 μL/well) can bind Trastuzumab with a linear range of 0.2–3 ng/mL. Image Credit: ACROBiosystems
Her2 antigen-binding affinity was determined by SPR using Trastuzumab (Herceptin®)
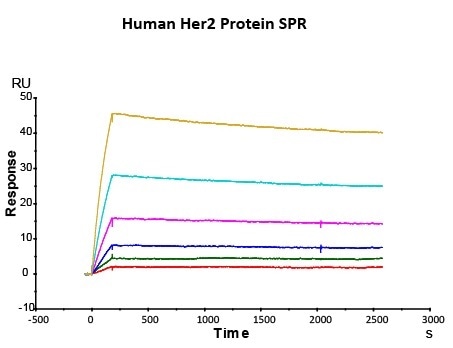
Immobilized Herceptin on CM5 Chip via anti-human Fc IgG, can bind Human Her2 Protein (Cat. No. HE2-H5225) with an affinity constant of 0.147 nM as determined in SPR assay (Biacore T200). Image Credit: ACROBiosystems
Her2 antigen binding affinity was determined by BLI using Trastuzumab (Herceptin®)
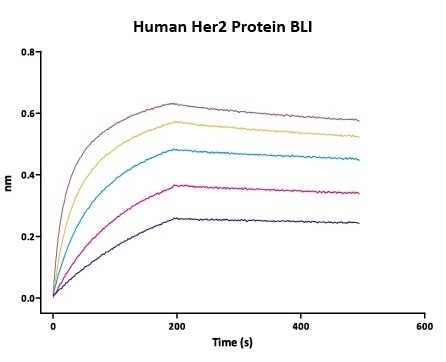
Immobilized Herceptin on AHC Biosensor can bind Human Her2 Protein (Cat. No. HE2-H5225) with an affinity constant of 0.93 nM as determined in BLI assay (Fortebio Octet 96). Image Credit: ACROBiosystems
High batch-to-batch consistency
ACROBiosystems strictly ensures the quality to deliver products with consistent performance. The newly produced products are subjected to side-by-side comparisons with the company’s internal standards in different types of assays. Only the products within the acceptable margin of difference are permitted for release.
Batch consistency of Her2 antigen was determined by ELISA using Trastuzumab (Herceptin®)
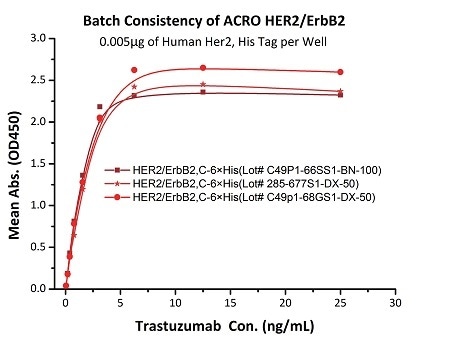
The binding activity of three different lots of hHER2 (Cat. No. HE2-H5225) was evaluated in the above ELISA analysis against Trastuzumab (Herceptin®). The result showed that the batch variation among the tested samples is negligible. Image Credit: ACROBiosystems
Batch consistency of TNF-alpha was determined by cytotoxicity assay
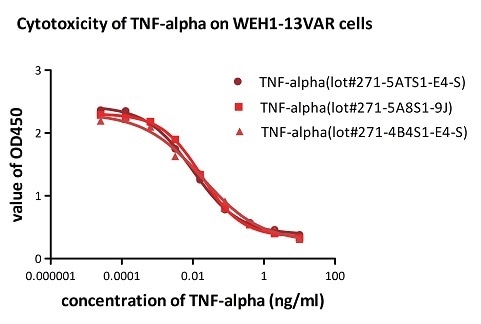
Recombinant Human TNF-alpha (Cat. No. TNA-H4211) induces cytotoxicity effect on the WEH1-13VAR cells in the presence of the metabolic inhibitor actinomycin D. The ED50 for this effect is 0.007–0.014 ng/mL. The result shows that the batch variation among the tested samples is negligible. Image Credit: ACROBiosystems
Batch consistency of FcRn was determined by SPR
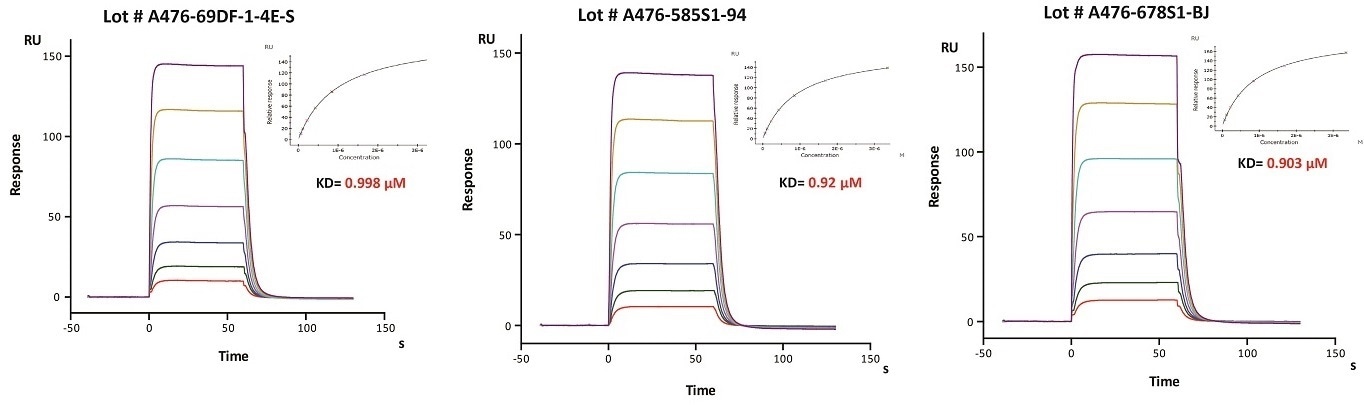
Immobilized Herceptin on CM5 Chip can bind Human FcRn / FCGRT & B2M Heterodimer Protein (Cat. No. FCM-H5286) with an affinity constant of about 0.9 μM as determined in SPR assay (Biacore 8K). The result shows that the batch variation among the tested different lots is negligible. Image Credit: ACROBiosystems
High stability and high activity
To maintain the stability of proteins, ACROBiosystems performs stability tests via accelerated and long-term stability testing. Post testing, the protein retains its quality and there is no evident activity loss across the life span of the products.
Stability of Her2 under 37 ℃ was determined by SDS-PAGE and ELISA
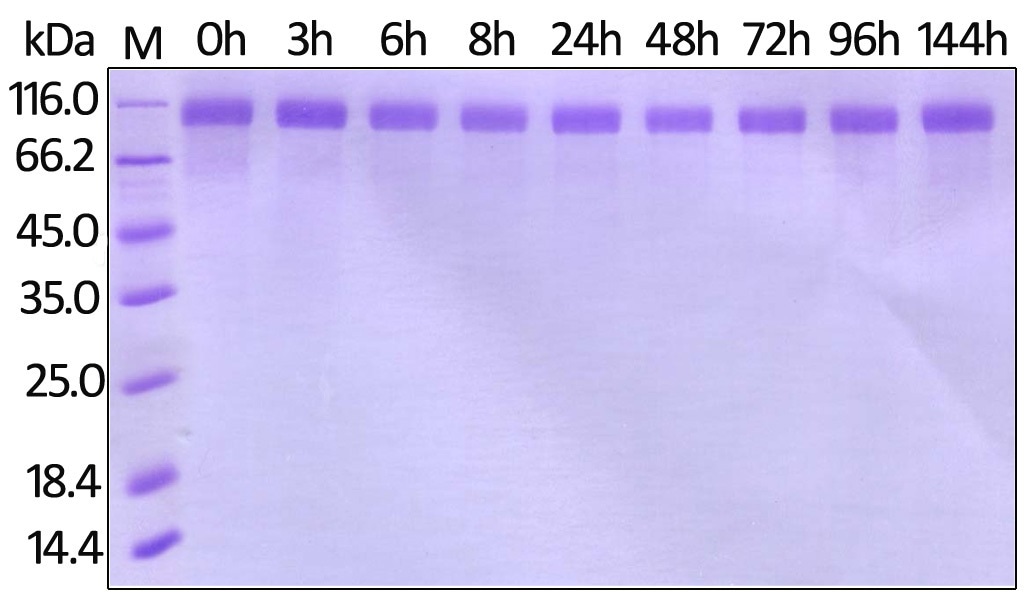
Human Her2, His Tag (Cat. No. HE2-H822R ) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. Image Credit: ACROBiosystems
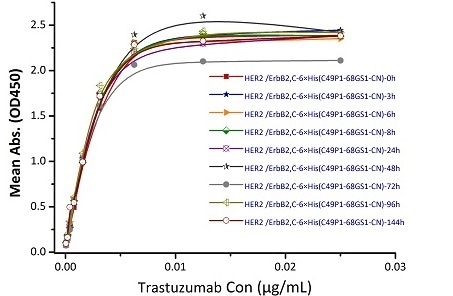
Immobilized Biotinylated Human Her2, His Tag (Cat. No. HE2-H822R) at 0.05 μg/mL (100 μL/well) can bind Trastuzumab. The result shows that the Biotinylated Human Her2, His Tag (Cat. No. HE2-H822R) is stable at 37 ℃ for 144 hours without performance reduction. Image Credit: ACROBiosystems
High purity and high quality
To fulfill the high purity needs of pharmaceutical applications, most of the proteins from ACROBiosystems are subjected to both SDS-PAGE and HPLC analyses. Only the ones complying with all requirements are issued a lot-specific certificate of assurance and are released.
SDS-PAGE
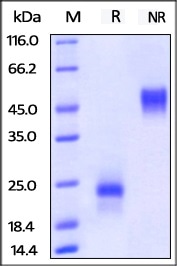
ActiveMax® Human VEGF165 (Cat. No. VE5-H4210) on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 98%. Image Credit: ACROBiosystems
References
- European Medicines Agency. Guideline on Similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1), 2014 [accessed 15.05.15]
- U.S. Department of Health and Human Services. Food and Drug Administration. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. Available at: (last accessed 10 November 2015)
- Bui, L.A. et al. Key considerations in the preclinical development of biosimilars, Drug Discov Today (2015)
- Al-Sabbagh A. et al. Development of biosimilars, Seminars in Arthritis and Rheumatism (2016)