Sino Biological's main strength resides in its recombinant expression platforms and proprietary mammalian cell culture. Such platforms make use of numerous proprietary reagents that are optimized uniquely to improve the expression levels of recombinant antibody in 293 and CHO cells.
Sino Biological offers a range of packages for customers who are in need of recombinant antibody expression services. Recombinant antibodies can be produced in an extensive range of formats, such as full-length, Fab, scFv, sdAb/VHH, chimeric, Fc fusion protein and bispecific antibodies.
Regarding antibody (or VH/VL) sequence information, cDNA or hybridoma cells, Sino Biological has the potential to provide high-quality recombinant antibodies with low endotoxin levels and high purity, together with elaborate project reports consisting of detailed QC data.
Service highlights
- >99% Success rate: Years of expertise and experience guarantee the maximum possible yields.
- Scalable production: Adaptable manufacturing scales varying from μg to grams.
- Fast turnaround time: Can be delivered in 10 days from gene sequences to purified recombinant antibodies.
- Customized specifications: High purity >95% validated by HPLC, Low endotoxin level, and high affinity validated by SPR and BLI assay.
- Diverse antibody formats: scFv, Fab, VHH, bispecific, chimeric, Fc-fusion protein, or full-length IgG, IgA, IgM, and IgE antibodies.
- In-house expression platforms: Proprietary expression vectors, transfection reagents, and culture media.
Recombinant antibody manufacturing services
High-throughput antibody production service
Timeline: 2 to 3 weeks
Generates thousands of recombinant antibodies per batch for antibody drug screening of users.
Small to mid-scale antibody production service
Timeline: 4 to 6 weeks
Provides high-quality antibodies for small-scale applications.
Large-scale antibody production service
Timeline: 6 to 10 weeks
Supplies large amounts of recombinant antibodies (grams) to fulfill industrial requirements of users.
Chimeric antibody production service
Timeline: 3 to 6 weeks
Integrates variable domains of users with human constant domains prior to antibody humanization.
Antibody fragment production service
Timeline: 3 to 6 weeks
Produces high-purity antibodies in any format (VHH, scFv, Fab, etc.) at inexpensive prices.
Bispecific antibody production service
Timeline: 3 to 6 weeks
Generates recombinant bsAbs in various formats.
The complete process for custom recombinant antibody production consists of gene synthesis with vector construction, codon optimization, purification, expression and QC testing. Users simply need to provide Sino Biological with the antibody (or VH/VL) sequence that they would like to express, and the company will take care of the rest.
Sino Biological’s recombinant antibody production services can also be adjusted for antibody subtype or host specificity to produce several types of antibodies, as listed below.
| |
Human |
Mouse |
Rat |
Rabbit |
Canine |
| Available IgGs |
IgG1/2/3/4 |
IgG1/2a/2b/3 |
IgG1/2a/2b/2c |
IgG |
IgG |
Case study of recombinant antibody production
Mouse Recombinant IgG (IgG1/IgG2a/IgG2b) Antibody Expression in HEK293 Cells
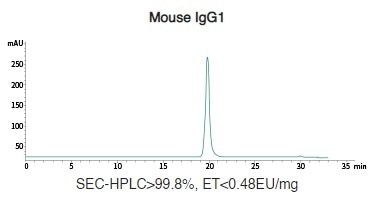
Image Credit: Sino Biological Inc
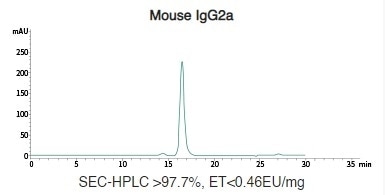
Image Credit: Sino Biological Inc
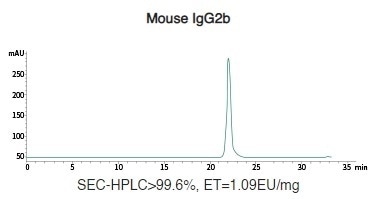
Image Credit: Sino Biological Inc
Facilities

Image Credit: Sino Biological Inc

Image Credit: Sino Biological Inc

Image Credit: Sino Biological Inc