Over the past 30 years, enzyme-linked immunosorbent assay (ELISA) has become a ubiquitous tool across various research fields. It has numerous applications, from screening for human immunodeficiency virus (HIV) and SARS-CoV-2 antibodies to detecting environmental and food contaminants.
This article explores enzyme-linked immunosorbent assays (ELISA) and their importance across many applications.
ELISA overview
ELISA is a method utilized to quantitatively detect an antigen (such as a foreign substance or toxin) within a specimen.
Typically, ELISAs are conducted on microplates, where the bottom of the microplate functions as the solid surface to which the relevant antigen attaches directly or via an antibody.
Researchers commonly utilize ELISA microplate readers to read and analyze numerous plates concurrently, enabling accurate, high-throughput ELISA measurements.
ELISA has contributed to some of the most impressive scientific breakthroughs of the 21st century. The origin and evolution of ELISA are described below.
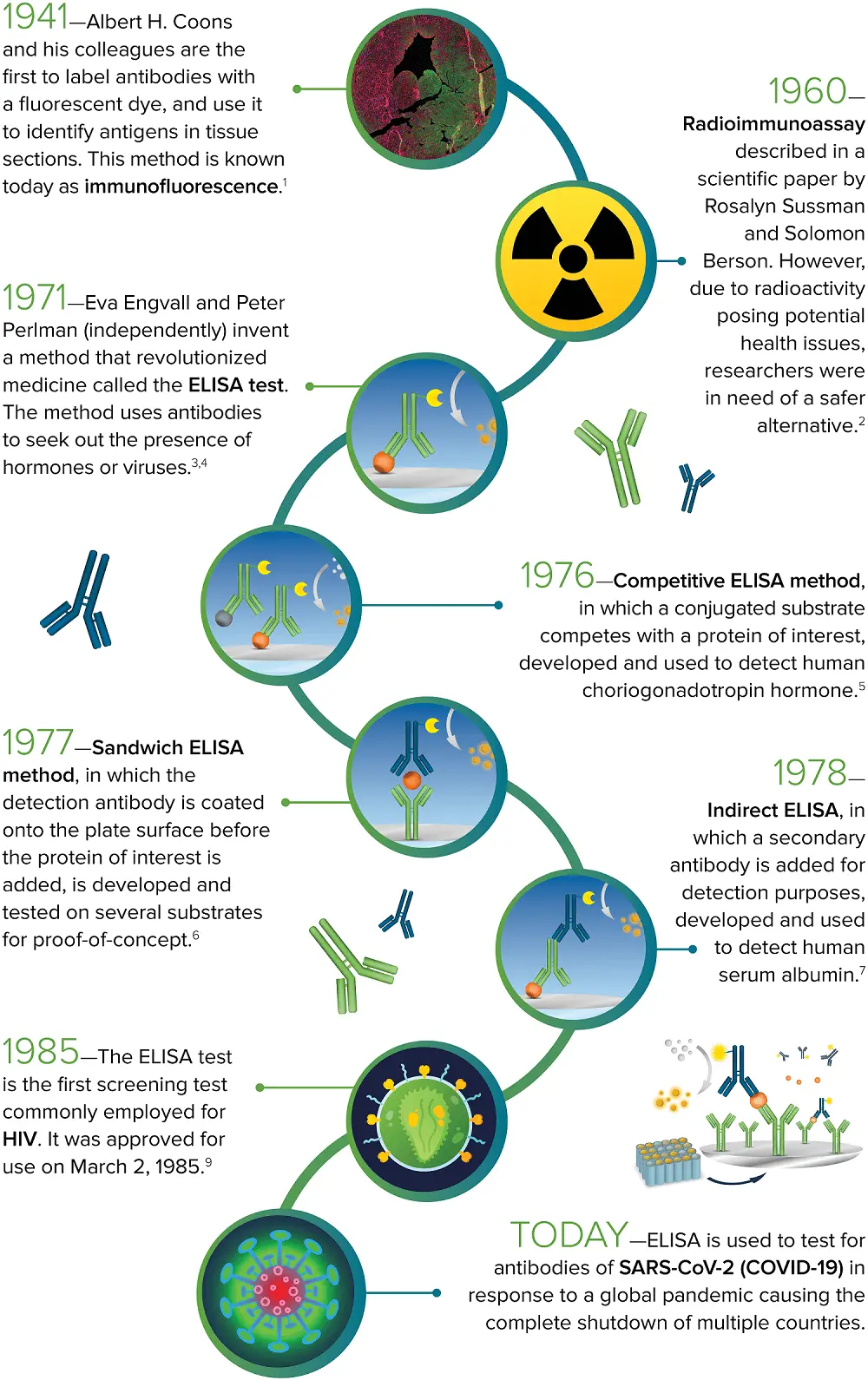
Image Credit: Molecular Devices UK Ltd
ELISA timeline
1941 – Albert H. Coons et al. are the first to label antibodies with a fluorescent dye and use the fluorescent-labeled antibodies to detect antigens in tissue sections. This technique is known now as immunofluorescence.1
1960 – Solomon Berson and Rosalyn Sussman described the radioimmunoassay in a scientific paper. However, concerns regarding the potential health risks of radioactivity prompted researchers to find a safer alternative.2
1971 – Peter Perlman and Eva Engvall develop a revolutionary method called the ELISA test. The ELISA uses antibodies to identify the presence of hormones or viruses.3,4
1976 – The competitive ELISA, in which a conjugated substrate competes with a protein of interest, was introduced and employed to detect human choriogonadotropin hormone.5
1977 – The sandwich ELISA, in which the detection antibody is coated onto the plate surface before the addition of the protein of interest, was introduced and tested on various substrates for proof-of-concept.6
1978 – Indirect ELISA, in which a secondary antibody is introduced for detection, was developed and utilized to identify human serum albumin.7
1985 – The ELISA is the first screening test frequently used for HIV. It was approved for use on the 2nd of March, 1985.9
Today – ELISA plays a crucial role in testing for antibodies of SARS-CoV-2 (COVID-19) in response to a global pandemic that caused multiple countries to shut down.

Image Credit: Molecular Devices UK Ltd
References and further reading
- Coons, A. H. The beginnings of immunofluorescence. J. Immunol. 87, 499–503 (1961).Coons, A. H. The beginnings of immunofluorescence. J. Immunol. 87, 499–503 (1961).
- Yalow, Rosalyn and Berson, Solomon. “Immunoassay of endogenous plasma insulin in man.” The Journal of Clinical Investigation. 1960;39: 1157–75.Yalow, Rosalyn and Berson, Solomon. “Immunoassay of endogenous plasma insulin in man.” The Journal of Clinical Investigation. 1960;39: 1157–75.
- Perlmann, Peter et al. “Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G.” Immunochemistry. 1971;8 (9): 871–4.Perlmann, Peter et al. “Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G.” Immunochemistry. 1971;8 (9): 871–4.
- Schuurs, A. “Immunoassay using antigen—enzyme conjugates.” FEBS Letters. 1971;15 (3): 232–236.Schuurs, A. “Immunoassay using antigen—enzyme conjugates.” FEBS Letters. 1971;15 (3): 232–236.
- Yorde, Donald et al. “Competitive Enzyme-Linked Immunoassay with Use of Soluble Enzyme/Antibody Immune Complexes for Labeling. I. Measurement of Human Choriogonadotropin.” Clin. Chem. 1976;22/8,1372–1377 Yorde, Donald et al. “Competitive Enzyme-Linked Immunoassay with Use of Soluble Enzyme/Antibody Immune Complexes for Labeling. I. Measurement of Human Choriogonadotropin.” Clin. Chem. 1976;22/8,1372–1377
- Kato, K et al. “Use of rabbit antibody IgG bound onto plain and aminoalkylsilyl glass surface for the enzyme-linked sandwich immunoassay.” J Biochem. 1977 Jul;82(1):261–6.Kato, K et al. “Use of rabbit antiboty IgG bound onto plain and aminoalkylsilyl glass surface for the enzyme-linked sandwich immunoassay.” J Biochem. 1977 Jul;82(1):261–6.
- Lindström, P et al. “IgG autoantibody to human serum albumin studied by the ELISA-technique.” Scand J Immunol. 1978;7(5):419-25.Lindström, P et al. “IgG autoantibody to human serum albumin studied by the ELISA-technique.” Scand J Immunol. 1978;7(5):419-25.
- Czerkinsky, C et al. “A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells.” J Immunol Methods. 1983;65 (1–2): 109–121.Czerkinsky, C et al. “A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells.” J Immunol Methods. 1983;65 (1–2): 109–121.
- Alexander, Thomas. “Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution.” Clinical and Vaccine Immunology. 2016 Apr;23(4):249-253.Alexander, Thomas. “Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution.” Clinical and Vaccine Immunology. 2016 Apr;23(4):249-253.
About Molecular Devices UK Ltd
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.