Seasonal flu, a common infectious disease of the respiratory tract, is caused by the influenza virus. There are four kinds of influenza virus, influenza type A, B, C, and D.
As far as the medical community is concerned, the A and B types are of great interest because they can cause seasonal epidemics. Influenza A, such as the A/California/04/09 (H1N1) and A/Hong Kong/1/1968 (H3N2), could even result in global pandemics.
To avoid flu infection, vaccination is the most efficient method. On an annual basis, different flu strains are chosen as vaccine candidates depending on surveillance data of the new isolates and the vaccines’ performance from the earlier season.
In recent years, most influenza vaccines have been quadrivalent or trivalent, made of one H1N1, one H3N2, and either one or two type B viruses (Victoria lineage and Yamagata).
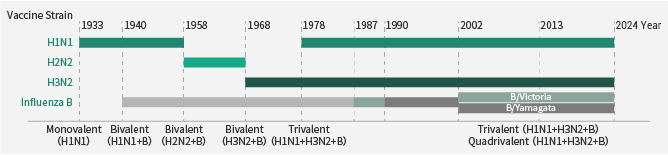
Image Credit: Sino Biological Inc
Sino Biological provides recombinant flu antigen products under its ProVir® viral antigen collection. The product line spans Neuraminidase (NA), Hemagglutinin (HA), and Nucleoprotein (NP) proteins from all WHO-recommended vaccine strains in the past few years.
Such antigens could be utilized to examine vaccine-induced antibody response. Sino Biological also has many monoclonal antibodies against the flu antigens. Such reagents could help streamline appropriate assay development.
Year 2023–2024
Table 1. Source: Sino Biological Inc
| . |
. |
A/Victoria/4897/2022 (H1N1)
Recommended for Egg-based (quadrivalent,trivalent) Vaccine
HA: 40938-V08H (pre-order), 40938-V08B (pre-order)
NA: 40939-V08B (pre-order), 40941-V08B (pre-order) |
A/Darwin/9/2021 (H3N2)
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40859-V08B, 40859-V08H
NA: 40860-V08B
NP: 40858-V08B |
B/Austria/1359417/2021 (B/Victoria lineage)
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40862-V08B, 40862-V08H
NA: 40863-V08B
NP: 40861-V08B |
A/Wisconsin/67/2022 (H1N1)
Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40940-V08H (pre-order), 40940-V08B (pre-order)
NA: 40941-V08B (pre-order)
NP: 40942-V08B (pre-order) |
B/Phuket/3073/2013 (B/Yamagata lineage)
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent) Vaccine
HA: 40498-VNAB, 40498-V08B, 40498-V08H1
NA: 40502-V07B
NP: 40500-V08B |
|
Year 2022–2023
Table 2. Source: Sino Biological Inc
| . |
. |
A/Victoria/2570/2019 (H1N1)
Recommended for Egg-based (quadrivalent,trivalent) Vaccine
HA: 40787-V08H, 40787-V08H1
NA: 40785-V08B
NP: 40774-V08B |
A/Wisconsin/588/2019 (H1N1)
Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40787-V08H, 40787-V08H1
NA: 40785-V08B
NP: 40774-V08B |
B/Phuket/3073/2013
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent) Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |
A/Darwin/9/2021 (H3N2)
Recommended for Egg-based (quadrivalent, trivalent) Vaccine
HA: 40859-V08H, 40859-V08B
NA: 40860-V08B
NP: 40858-V08B |
B/Austria/1359417/2021 (B/Victoria lineage)
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40862-V08H, 40862-V08B
NA: 40863-V08B
NP: 40861-V08B |
A/Darwin/6/2021 (H3N2)
Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40868-V08H, 40868-V08B
NA: 40869-V08B
NP: 40858-V08B |
Year 2021–2022
Table 3. Source: Sino Biological Inc
| . |
. |
A/Victoria/2570/2019 (H1N1)
Recommended for Egg-based (quadrivalent,trivalent) Vaccine
HA: 40787-V08H, 40787-V08H1
NA: 40785-V08B
NP: 40774-V08B |
A/Wisconsin/588/2019 (H1N1)
Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40787-V08H, 40787-V08H1
NA: 40785-V08B
NP: 40774-V08B |
B/Washington/02/2019
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40722-V08H
NA: 40790-V08B
NP: 40755-V08B |
B/Phuket/3073/2013
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent) Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |
A/Cambodia/E0826360/2020 (H3N2)
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40789-V08H, 40789-V08H1
NA: 40784-V08B
NP: 40778-V08B |
|
Year 2020–2021
Table 4. Source: Sino Biological Inc
| . |
. |
A/Guangdong-Maonan/SWL1536/2019 (H1N1)
Recommended for Egg-based (quadrivalent, trivalent) Vaccine
HA: 40717-V08H
NP: 40723-V08B |
A/Hong Kong/2671/2019 (H3N2)
Recommended for Egg-based (quadrivalent, trivalent) Vaccine
HA: 40721-V08H
NP: 40753-V08B |
A/Hawaii/70/2019 (H1N1)
Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40717-V08H
NP: 40724-V08B |
A/Hong Kong/45/2019 (H3N2)
Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40765-V08H
NP: 40754-V08B |
B/Phuket/3073/2013
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent) Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |
B/Washington/02/2019
Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine
HA: 40722-V08H
NA: 40790-V08B
NP: 40755-V08B |
Year 2019–2020
Table 5. Source: Sino Biological Inc
| . |
. |
A/Brisbane/02/2018 (H1N1)
Recommended for Egg-based (quadrivalent) Vaccine
HA: 40719-V08H
NA: 40767-V08B
NP: 40776-V08B |
A/Kansas/14/2017 (H3N2)
Recommended for Egg-based (quadrivalent) Vaccine
HA: 40720-V08H
NA: 40766-V08B
NP: 40779-V08B |
B/Phuket/3073/2013
Recommended for Egg-based (quadrivalent) Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |
B/Colorado/06/2017
Recommended for Egg-based (quadrivalent); trivalent Vaccine
HA: 40581-V08H
NP: 40782-V08B |
Year 2018–2019
Table 6. Source: Sino Biological Inc
| . |
. |
A/Michigan/45/2015 (H1N1)
Recommended for quadrivalent Vaccine
HA: 40567-V08H1
NA: 40568-V08B
NP: 40777-V08B |
A/Singapore/INFIMH-16-0019/2016 (H3N2)
Recommended for quadrivalent Vaccine
HA: 40580-V08H
NP: 40779-V08B |
B/Phuket/3073/2013
Recommended for quadrivalent Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |
B/Colorado/06/2017
Recommended for quadrivalent; trivalent Vaccine
HA: 40581-V08H
NP: 40782-V08B |
Year 2017–2018
Table 7. Source: Sino Biological Inc
| . |
. |
A/Michigan/45/2015 (H1N1)
Recommended for trivalent Vaccine
HA: 40567-V08H1
NA: 40568-V08B
NP: 40777-V08B |
A/Hong Kong/4801/2014 (H3N2)
Recommended for trivalent Vaccine
HA: 40555-V08B
NA: 40569-V08B
NP: 40781-V08B |
B/Brisbane/60/2008
Recommended for trivalent Vaccine
HA: 40016-V08B
NA: 40203-VNAHC
NP: 40783-V08B |
B/Phuket/3073/2013
Recommended for quadrivalent Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |
Year 2016–2017
Table 8. Source: Sino Biological Inc
| . |
. |
A/California/7/2009 (H1N1)
Recommended for trivalent Vaccine
HA: 11085-V08B
NP: 40205-V08B |
A/Hong Kong/4801/2014 (H3N2)
Recommended for trivalent Vaccine
HA: 40555-V08B
NA: 40569-V08B
NP: 40781-V08B |
B/Brisbane/60/2008
Recommended for trivalent Vaccine
HA: 40016-V08B
NA: 40203-VNAHC
NP: 40783-V08B |
B/Phuket/3073/2013
Recommended for quadrivalent Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |
Year 2015–2016
Table 9. Source: Sino Biological Inc
| . |
. |
A/California/7/2009 (H1N1)
Recommended for trivalent Vaccine
HA: 11085-V08B
NP: 40205-V08B |
A/California/7/2009 (H1N1)
Recommended for trivalent Vaccine
HA: 11085-V08B
NP: 40205-V08B |
B/Brisbane/60/2008
Recommended for quadrivalent Vaccine
HA: 40016-V08B
NA: 40203-VNAHC
NP: 40783-V08B |
B/Phuket/3073/2013
Recommended for trivalent Vaccine
HA: 40498-V08B
NA: 40502-V07B
NP: 40500-V08B |