In the realm of in vitro three-dimensional (3D) culture technologies, particularly organoids, recent advancements have brought about significant changes in the creation of more physiologically relevant models for human cancer.
These models play a vital role in translating fundamental cancer research into effective treatment approaches for individuals diagnosed with cancer.1
A notable benefit associated with organoids is their ability to be cultured from healthy tumor tissues sourced from individual patients. This approach allows medical professionals to conduct personalized drug testing tailored to each patient, thereby generating results that contribute to the formulation of individualized treatment plans.
Cancer drug screening has traditionally relied on two-dimensional (2D) cell-culture models and patient-derived tumor xenograft (PDX) models.
Despite their merits, these models fall short of capturing the heterogeneity and intricacies of tumors within a 3D framework. Additionally, PDX models are expensive and consume a significant amount of time, placing constraints on their widespread application.2-4
The advancement of tumor organoids has transformed cancer studies by offering more physiological and individualized models for researching cancer.
With their ability to replicate the tumor microenvironment effectively, their potential for testing drugs tailored to patients, and their benefits over standard models, organoids present a hopeful method for enhancing strategies in cancer treatment.5
This article describes organoids' features and their role in cancer research.
Breakthroughs for the use of organoid models
In 2009, Sato et al.6 outlined the ability to continuously cultivate 3D organoids in the Corning® Matrigel® matrix for organoid culture (Corning Inc., US) from individual Lgr5-positive intestinal stem cells in controlled conditions that artificially supply specific factors (such as R-spondin, noggin, epidermal growth factors).
Intestinal organoids were established, and in the subsequent five years, various protocols for organoid culture emerged for the colon, small intestine, retina, brain, liver, stomach, and breast.7-8
In 2014, Gao et al. and Li et al.9 made significant strides in utilizing organoid models for cancer research.
Their comprehensive genomic analysis demonstrated that the organoids closely mirrored in vivo prostate cancers in terms of copy-number alterations, gene-expression subtypes, and histological patterns.
Following this, researchers effectively generated organoid models for colorectal cancer,10 gastric cancer,11 prostate cancer,12 bladder tumors,13 esophageal cancer, endometrial cancer, and other types of tumors.14-15
Establishment of numerous organoid culture methods from various organs. Image Credit: Sino Biological Inc.
Cell-culture models
Tumor organoids offer a more precise representation of the tumor microenvironment compared with conventional tumor research models. They not only visually depict the tumor growth process but also capture individual variations among patients.
Additional benefits include cells that closely resemble physiological conditions, a consistent genome, and suitability for both biological transfection and high-throughput screening (Table 1). In general, tumor organoids provide an organ-level research system that is reliable, intuitive, effective and avoids ethical disagreements.
Table 1. Comparison of three major models. Source: Sino Biological Inc.
| |
Animal Models |
2D Cell Culture |
3D Organoids |
| High-throughput |
Limited |
Yes |
Yes |
| Manipulability |
Limited |
Excellent |
Good, but may have experimental variability |
| Biobanking |
Only at the cellular level |
Yes |
Yes |
| Gene editing |
Requires generation of embryonic stem cells |
Yes |
Yes |
| Modeling organogenesis |
Limited by complex tissue environment |
Lack cell-cell and cell-matrix interactions |
Suitable for studying cell-cell communication; reduced complexity |
| Modeling human development and disease |
Yes |
Poor |
Yes |
Importance of cytokines in organoid culture
To successfully cultivate organoids, specific elements like Matrigel® matrix and culture media are crucial, along with essential cytokines.
These cytokines fall into categories: activators, inhibitors, and hormones that facilitate cell growth, differentiation, and signaling pathways; cytokines that promote cell proliferation; and cytokines that enhance the success rate of organoid culture.
Various organoid cultures survive in a medium supplemented with growth factors relevant to the specific organ of interest.16-17
For instance, mouse small-intestinal organoid cultures can survive in a medium enriched with noggin, R-spondin 1 or R-spondin 3, and epidermal growth factor (EGF), often negating the need for additional Wnt since Paneth cells in culture produce ample Wnt for organoid proliferation.18
As a leading global supplier of high-quality recombinant cytokines, Sino Biological has created a range of recombinant growth factors with exceptional bioactivity, consistent batch-to-batch quality, and purity to enable optimal and reliable organoid growth. The applications of these growth factors in organoid culture are detailed in Table 2.
Table 2. Growth factors for organoid culture. Source: Sino Biological Inc.
| Organoid Type |
Growth Factors |
| Intestinal organoid (enteroid) |
EGF, noggin, R-spondin1, FGF2, FGF4, activin A, BMP4, IGF1 |
| Gastric organoid |
EGF, noggin, R-spondin1, FGF10, FGF4, activin A, BMP4 |
| Brain organoid |
FGF2, TGF-β, insulin, Shh, BDNF, GDNF, FGF8, BMP7 |
| Liver organoid |
EGF, noggin, R-spondin1, FGF2, activin A, FGF4, BMP4, HGF, TGF-α, insulin, BMP2 |
| Lung organoid |
EGF, noggin, R-spondin1, FGF2, FGF10, activin A, FGF4, BMP4 |
| Kidney organoid |
BMP2, BMP4, BMP7, FGF2, FGF9 |
| Pancreatic organoid |
EGF, noggin, R-spondin1, FGF10 |
| Prostate organoid |
EGF, noggin, R-spondin1, activin A, FGF2, FGF10 |
| Breast organoid |
EGF, R-spondin1, R-spondin2, noggin, FGF2, FGF10 |
| Inner-ear organoid |
BMP4, FGF2 |
| Retinal organoid |
Shh |
Featured recombinant growth factor proteins
Human recombinant EGF protein (ECD), HPLC-verified | Cat: GMP-10605-HNAE
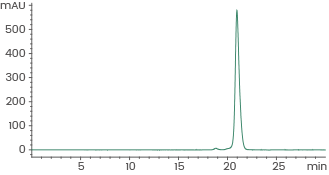
≥ 95 % as determined by SEC-HPLC. Image Credit: Sino Biological Inc.
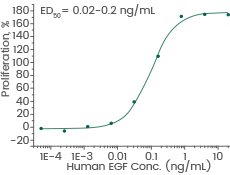
Cell proliferation assay using Balb/C 3T3 mouse embryonic fibroblasts. Image Credit: Sino Biological Inc.
Human recombinant Noggin/NOG protein, HPLC-verified | Cat: 10267-HNAH
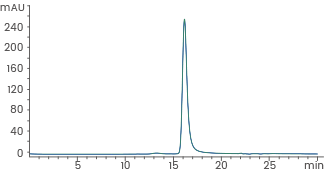
≥ 95 % as determined by SEC-HPLC. Image Credit: Sino Biological Inc.
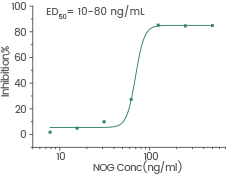
Measured by its ability to inhibit BMP4-induced alkaline phosphatase production by MC3T3E1 mouse preosteoblast cells. Image Credit: Sino Biological Inc.
Human recombinant FGF2 protein | Cat: GMP-10014-HNAE
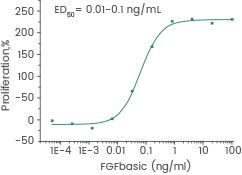
Measured in a cell proliferation assay using Balb/c 3T3 mouse embryonic fibroblasts. Image Credit: Sino Biological Inc.
Organoid applications in cancer research
Organoids have become an essential 3D model in cancer research as they can replicate various elements of the intricate structure and function seen in the corresponding tissue in vivo. The utilization of organoids should take into account the tumor organoid biobank, screening for new drugs, and the realm of precision medicine.
Tumor organoid biobank
The tumor organoid biobank proves useful for conducting drug sensitivity testing, drug screening, and toxicity assessments based on the heterogeneity of tumors. An illustrative example is breast cancer, characterized by its significant heterogeneity and multiple pathological subtypes.
Sachs et al.19 established 95 breast cancer organoids from 155 breast cancer specimens, achieving a success rate exceeding 80 %. The distribution of pathological types in this organoid biobank aligns with the epidemiology of breast cancer, including 50 % to 80 % invasive ductal carcinoma and 5 % to 15 % invasive lobular carcinoma.
Whole-genome sequencing and transcriptomic sequencing have also indicated that organoids maintain high similarity to parent tumors, in terms of copy-number variations and gene-expression profiles, even after long-term culture.
Glioblastoma, the most prevalent brain tumor in adults, is exceptionally aggressive and displays robust resistance to targeted and immunotherapy.20
Jacob et al.21 established 70 organoids from 53 patients with glioblastoma. Analyses involving transcriptome, whole-exome, and single-cell transcriptome sequencing have demonstrated that glioblastoma organoids effectively preserve intra- and inter-tumor heterogeneity.
Building on this foundation, the research team co-cultivated glioblastoma organoids with chimeric antigen receptor (CAR) T cells, investigated their response to CAR-T-cell therapy, and uncovered the potential application of tumor organoids in immunotherapy.
New drug screening
Organoids serve as excellent models for drug screening due to their perpetual cell proliferation capacity. However, these immortalized cells often fall short of representing the phenotypes and lose the genetic diversity of the original tumor over prolonged cultivation, which can increase the failure rate in clinical drug screening trials.25
Hans Clevers et al.22 established, for the first time, a patient-derived colorectal tumor organoids biobank that corresponds to normal tissues. They conducted high-throughput drug screens (including 10 chemotherapy drugs and 25 clinical drugs) to assess 83 drug combinations.
The study encompassed 29 drugs in clinical trials and 29 compounds targeting distinct cancer targets. Notably, the research uncovered that cetuximab does not exhibit sensitivity in organoid responses for patients with BRAF mutations, while Nutlin-3a shows sensitivity in organoids with TP53 gene mutations.
In alignment with findings from a prior retrospective study of clinical patients, inhibitors of Wnt secretion were identified as having a potential role in patients with mutations in the negative Wnt feedback regulator RNF43.
Precision medicine
Organoids have demonstrated significant promise in the realm of precision medicine, which aims to customize medical treatments for individual patients based on their unique genomics and metabolomics.23
By offering a more physiologically relevant and personalized representation of human organs or tissues, organoids prove valuable in forecasting individual patient reactions to drugs and other therapeutic interventions.
This methodology brings advancements in assay speed, reproducibility, standardization, and automation, which are essential to realize the translational potential of patient-derived organoids as clinical tools.24
Conclusion
Given the significant drawbacks in 2D cell cultures and animal models, it is evident that 3D organoid systems offer novel opportunities for developing organ mechanisms that can tackle crucial challenges in biomedical industries.
Organoids also exhibit substantial potential for diverse translational applications, including regenerative medicine and gene editing,5 thus transforming in vitro culture tools for biomedical research. Several hurdles persist in the development of 3D organoids which need to be overcome.
These challenges include regulating self-organization to generate well-developed organoids, mitigating the considerable diversity in organoid cultures, enhancing the relevance of shapes and sizes to physiological norms, extending the lifespan of organoids to achieve maturity, and reproducing the intricate biological complexity of native organs by integrating major biological compartments such as vasculature and nervous systems.
Overcoming these persistent obstacles will ultimately pave the way for more approved organoids for application in clinical trials.
References and further reading
- Li L, Knutsdottir H, Hui K, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019;4(2):e121490. Published 2019 Jan 24. doi:10.1172/jci.insight.121490
- Gillet JP, Varma S, Gottesman MM. The clinical relevance of cancer cell lines. J Natl Cancer Inst. 2013;105(7):452-458. doi:10.1093/jnci/djt007
- Byrne AT, Alférez DG, Amant F, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts [published correction appears in Nat Rev Cancer. 2017 Sep 15;:]. Nat Rev Cancer. 2017;17(4):254-268. doi:10.1038/nrc.2016.140
- Gao H, Korn JM, Ferretti S, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318-1325. doi:10.1038/nm.3954
- Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407-418. doi:10.1038/s41568-018-0007-6
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262-265. doi:10.1038/nature07935
- Chen HI, Song H, Ming GL. Applications of Human Brain Organoids to Clinical Problems. Dev Dyn. 2019;248(1):53-64. doi:10.1002/dvdy.24662
- Pompaiah M, Bartfeld S. Gastric Organoids: An Emerging Model System to Study Helicobacter pylori Pathogenesis. Curr Top Microbiol Immunol. 2017;400:149-168. doi:10.1007/978-3-319-50520-6_7
- Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1-2):324-338. doi:10.1016/j.cell.2014.12.021
- Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141(5):1762-1772. doi:10.1053/j.gastro.2011.07.050
- Yan HHN, Siu HC, Law S, et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell. 2018;23(6):882-897.e11. doi:10.1016/j.stem.2018.09.016
- Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176-187. doi:10.1016/j.cell.2014.08.016
- Lee SH, Hu W, Matulay JT, et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell. 2018;173(2):515-528.e17. doi:10.1016/j.cell.2018.03.017
- Li X, Francies HE, Secrier M, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun. 2018;9(1):2983. Published 2018 Jul 30. doi:10.1038/s41467-018-05190-9
- Girda E, Huang EC, Leiserowitz GS, Smith LH. The Use of Endometrial Cancer Patient-Derived Organoid Culture for Drug Sensitivity Testing Is Feasible. Int J Gynecol Cancer. 2017;27(8):1701-1707. doi:10.1097/IGC.0000000000001061
- Clevers H. Modeling Development and Disease with Organoids. Cell. 2016;165(7):1586-1597. doi:10.1016/j.cell.2016.05.082
- Hamilton JG, Banerjee SC, Carlsson SV, et al. Clinician perspectives on communication and implementation challenges in precision oncology. Per Med. 2021;18(6):559-572. doi:10.2217/pme-2021-0048
- Sachs N, de Ligt J, Kopper O, et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 2018;172(1-2):373-386.e10. doi:10.1016/j.cell.2017.11.010
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262-265. doi:10.1038/nature07935
- Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432-446. doi:10.1016/S0140-6736(18)30990-5
- Jacob F, Salinas RD, Zhang DY, et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell. 2020;180(1):188-204.e22. doi:10.1016/j.cell.2019.11.036
- Bose S, Clevers H, Shen X. Promises and Challenges of Organoid-Guided Precision Medicine. Med. 2021;2(9):1011-1026. doi:10.1016/j.medj.2021.08.005
- Boonekamp KE, Kretzschmar K, Wiener DJ, et al. Long-term expansion and differentiation of adult murine epidermal stem cells in 3D organoid cultures. Proc Natl Acad Sci U S A. 2019;116(29):14630-14638. doi:10.1073/pnas.1715272116
- Matano M, Date S, Shimokawa M, et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. 2015;21(3):256-262. doi:10.1038/nm.3802
- Ren X, Chen W, Yang Q, Li X, Xu L. Patient-derived cancer organoids for drug screening: Basic technology and clinical application. J Gastroenterol Hepatol. 2022;37(8):1446-1454. doi:10.1111/jgh.15930
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.