The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) put an unprecedented burden on the global healthcare system, with more than 425 million infections and 5.8 million deaths to date (March 2022). Regarding disease severity, a gender difference has been observed worldwide, with women developing less severe infections than men.

Social distancing due to COVID-19. Image Credit: Kzenon/Shutterstock.com
What is the COVID-19 pandemic?
An outbreak of COVID-19 was first detected in Wuhan city of China in December 2019. The causative pathogen of COVID-19 is known as SARS-CoV-2, an enveloped, positive-sense, single-stranded RNA virus belonging to the human beta-coronavirus family.
Although about 80% of COVID-19 patients develop only mild symptoms, the risk of severe infection is significantly higher among elderly people, comorbid patients, and immunocompromised patients.
A growing pool of evidence suggests that being male is also a risk factor for severe COVID-19. It has been estimated that the risk of death from COVID-19 is 20% higher in men than women. In addition, men are more prone than women to developing serious complications that require intensive care unit (ICU) admissions and mechanical ventilation.
Why are men more susceptible to severe COVID-19 than women?
There are many genetic, immunological, and lifestyle or behavioral factors that might increase the risk of disease severity in men.
Genetic susceptibility
Infection with SARS-CoV-2 is initiated by the binding of the viral spike protein to host cell receptor angiotensin-converting enzyme 2 (ACE2) present on respiratory epithelial cells. This is followed by the fusion of the viral envelope with the host cell membrane and the transportation of viral RNA into the host cell.
There is evidence indicating that the expression of ACE2 in the human lungs is directly proportional to the severity of SARS-CoV-2 infection. Thus, any living organisms that have higher expression of ACE2 in alveolar epithelial cells are expected to accelerate the viral entry to the respiratory tract.
In this context, single-cell RNA-sequencing analyses have shown that Asian men have significantly higher expression of ACE2 in the lungs than Asian women. Thus, genetic expression and cellular distribution pattern of ACE2 make men more susceptible to SARS-CoV-2 infection than women.
Immunological susceptibility
Women in general exhibit a higher immune response to viral or bacterial infection than men. This could be because women have two X chromosomes instead of the one observed in men.
The X chromosome is known to increase the expression of important immune components that help induce robust infection-controlling immune responses. In addition, female sex hormones including estrogen and progesterone play vital roles in triggering immune signaling and reducing inflammation, respectively.
There is evidence indicating that women produce more antibodies than men in response to influenza vaccination. This highlights their potency in inducing strong immune responses against invading pathogens. However, this special ability sometimes makes women more susceptible to developing autoimmune diseases wherein the body’s immune system starts attacking its own body cells/tissues mistakenly.
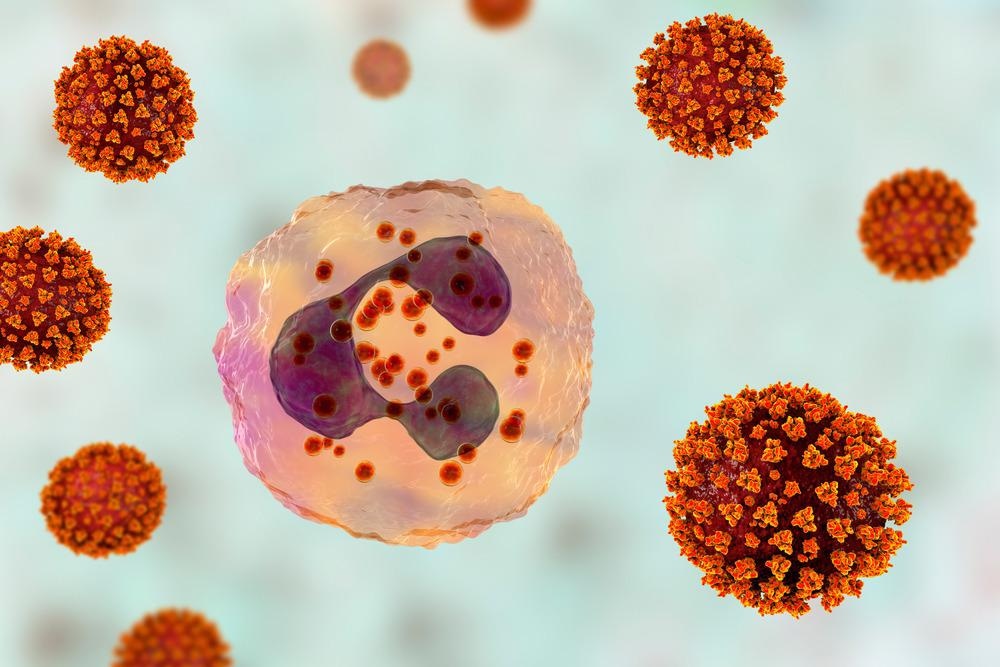
The innate immune system of the human body acts as the first-line of defense against any invading pathogens. As a part of the early innate immune response, controlled activation of interferon signaling leads to the production of pro-inflammatory cytokines and chemokines at the site of infection. A measured inflammatory response is vital for eliminating pathogens at the early infection stage.
In COVID-19 patients, hyperinflammation is regarded as the major hallmark of disease severity. In severe COVID-19 patients, uncontrolled activation of type 1 interferon response has been found to cause excessive production of cytokines (cytokine storm), leading to severe lung damage and multiorgan failure.
A recent study conducted on male and female patients with moderate COVID-19 has shown that male patients have higher plasma levels of pro-inflammatory cytokines and chemokines and higher activation of non-classical monocytes than female patients. The same study also highlighted that female patients have significantly higher activation of T cell response than male patients.
The poor T cell response in males is associated with a worse disease prognosis. However, the higher innate immune response in male patients is not associated with worse disease outcomes. In contrast, such responses make female patients more susceptible to developing severe COVID-19.
Given the study findings, the scientists suggest that male and female patients may be benefited from therapeutic interventions that are designed to induce T cell response and suppress an innate immune response, respectively.
SARS-CoV-2 viruses activating neutrophil, conceptual 3D illustration. Overactive neutrophils in COVID-19 are associated with hyperinflammation that drives lung and multi-organ damage. Image Credit: Kateryna Kon/Shutterstock.com
Lifestyle factors
A relatively higher rate of smoking and alcohol consumption has been observed among men globally. The disparity in these lifestyle behaviors between men and women could be a potential reason for gender-based susceptibility to COVID-19. In addition, men are more prone to high-risk behaviors than women, which further increases their chances to contract COVID-19.
Several studies have indicated that women exhibit higher adherence to COVID-19 related control measures, including social distancing, face mask-wearing, hand washing, and movement restrictions. These COVID-19 appropriate behaviors help protect people from contracting COVID-19.
Besides lifestyle and behavioral factors, certain occupational risk factors may also put men at higher risk for COVID-19. In low-skilled occupations like transportation, food processing, delivery, construction, and manufacturing, the number of male workers is significantly higher than female workers. Studies have shown that workers in these occupations are at higher risk of severe COVID-19 and mortality.
References
Further Reading